RESEARCH
Caloric Restriction and tissue resilience
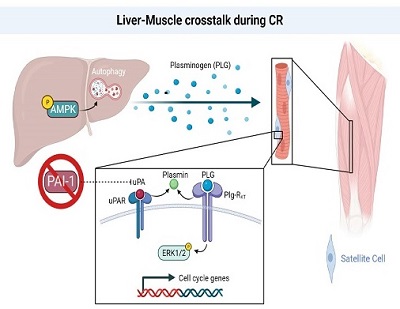
Caloric restriction (CR) without malnutrition has been shown to extend lifespan with associated increase in tissue resilience. In skeletal muscle, short-term (3 month) CR increases muscle stem cell (satellite cell) number and regenerative function in both young and old mice. Much of these beneficial effects have been hypothesized to be mediated by secreted proteins. However, the identity of these proteins is largely unknown due, in part, to limited detection methods of the protein secretome. To get around these limitations, we have used the Albumin-Cre/MetRS model to characterize the CR-associated liver secretome. We have identified several differentially expressed proteins including plasminogen, a multifunctional zymogen well known to regulate hemostasis and more recently shown to play a role in tissue regeneration, including skeletal muscle.
METRNL improves aged muscle regenerations
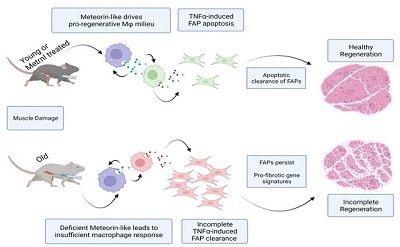
Meteorin-like (or METRNL) is a cytokine that is secreted by inflammatory immune cells during the early stages of the regenerative process. Our lab published evidence in Nature Metabolism in 2020 of the necessity of METRNL during muscle regeneration. We are actively expanding upon these findings by investigating chronic disease states that present with insufficient levels of METRNL in response to injury. We are keenly interested in the physiological loss of METRNL secretion by aged macrophage populations in the muscle and how the response to METRNL interacts with other interstitial cell types to combat pathological muscle regeneration.
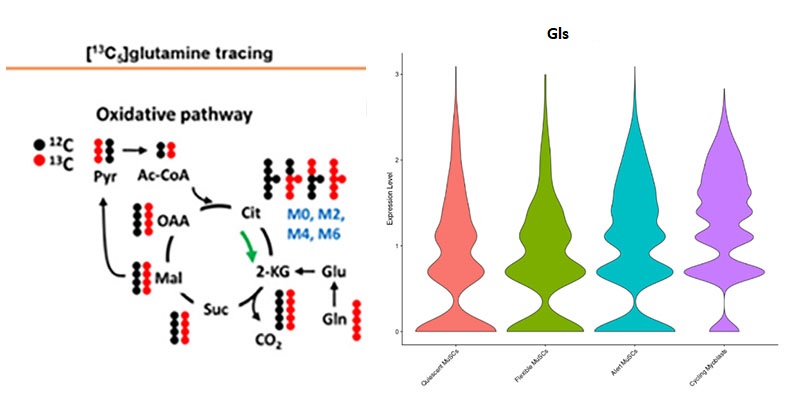
Muscle Stem (Satellite) Cell metabolism
Glutamine is one of the most active sources of carbon substrate used to generate energy in mitochondrial oxidative phosphorylation next to pyruvate and palmitate. We are actively investigating the important role of glutamine metabolism is the population of interstitial muscle stem cells that contribute to muscle’s regenerative capacity. By limiting metabolic shunting away from glutamine metabolism and enhancing the energy available to the muscle stem cell during its activation, we hope to maintain the resilience of muscle in models of aging.