More than a decade ago, DMPI faculty members Drs. Debbie Muoio and Tim Koves teamed with Drs. Robert Stevens, Olga Ilkayeva, and James Bain in the DMPI metabolomics lab to report on accumulation of incompletely oxidized lipid species (in the form of even-chain acylcarnitines) in skeletal muscle of obese and insulin resistant rodents and humans. These investigators then performed transgenic mouse studies to demonstrate a direct link between impaired mitochondrial processing of fatty acids and the insulin resistant state.
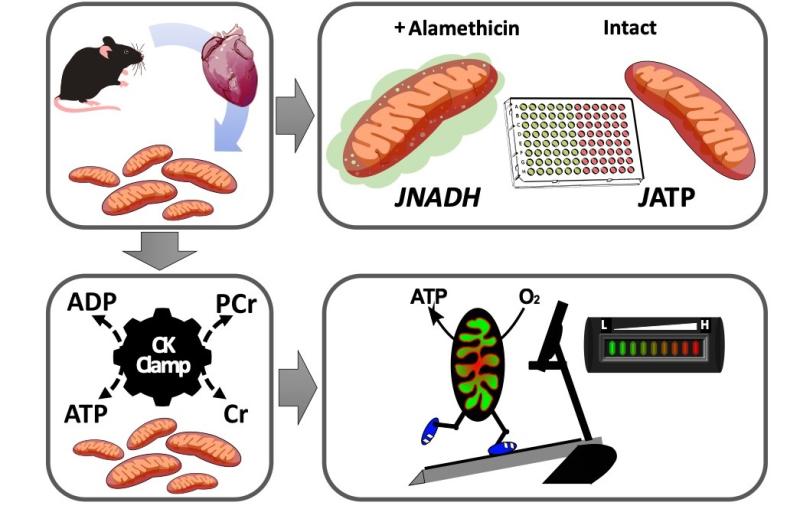
More recently, Drs. Muoio, Koves, and their team have developed a new and comprehensive system for evaluating mitochondrial power and efficiency under conditions of controlled energy demand. Unlike conventional respirometry methods, the new platform permits comprehensive assessment of respiratory fluxes (JO2) and energy transduction from carbon fuels to electron potential energy (NAD(P)H/NAD+ redox), membrane potential (DY), and ΔGATP. The creatine kinase clamp technique is used to control extramitochondrial ATP:ADP concentrations within the physiologic range. The system can be used to mimic an exercise “stress test” in which isolated mitochondria are exposed to a graded energetic challenge in the presence of different metabolic substrates. The platform allows measurement of up to 120 unique energy fluxes, and is being combined with mass spectrometry-based metabolomics, proteomics and metabolic flux analysis to evaluate mitochondrial remodeling in response to a variety of nutritional, pharmacological and genetic maneuvers relevant to metabolic disease.
Publications:
Fisher-Wellman K, Draper J, Davidson MT, Williams A, Narowski T, Slentz D, Ilkayeva O, Stevens RD, Wagner G, Hirschey M, Thompson JW, Olson DP, Kelly DP, Koves TR, Grimsrud PA, and Muoio DM. (2018) Respiratory phenomics across multiple models of mitochondrial protein hyperacylation reveals a paradoxically marginal impact on bioenergetics. Cell Reports, in press.
