RESEARCH
Developmental Biology of Adipose Tissue
Proper control of energy and nutrient homeostasis is crucial for growth, development, and the prevention of metabolic disease. Adipose tissue plays a critical role in the regulation of energy balance, functioning as a metabolic sensor that controls energy storage, energy utilization, and food intake. Pathological adipose tissue distribution and adipocyte dysfunction are intimately linked to obesity and obesity-associated diseases such as type 2 diabetes, cardiovascular disease, and cancer. The growing epidemic of obesity and rising costs associated with treating metabolic disease has increased the urgency for understanding all aspects of adipose tissue biology, including how adipocytes are formed throughout the body.

Ongoing Projects:
1. Transcriptional networks controlling the establishment and maintenance of white (energy-storing) and brown/beige (energy-burning) adipose tissue.
We have identified an important role for the C2H2 zinc-finger transcription factor, ZFP423, in adipose tissue development. ZFP423 regulates commitment of adipose precursor cells, or “preadipocytes” to the adipose lineage through activation of Pparg, the “master regulator” of adipocyte differentiation. Furthermore, we have revealed a critical role for ZFP423 in maintaining the cellular identity of white adipocytes through suppression of the thermogenic brown/beige adipocyte gene program. Remarkably, loss of genetic inactivation of Zfp423 in adult mice triggers a widespread conversion of white to beige-like adipocytes, leading to resistance or even reversal of diet-induced obesity. Ongoing efforts are focused on identifying pathways that regulate Zfp423 gene expression in the adipose lineage, as well as critical interacting partners and downstream effectors that mediate its function. Understanding the mechanisms that control Zfp423 expression and function in white adipocytes may lead to novel therapeutic strategies to combat obesity and metabolic disease.
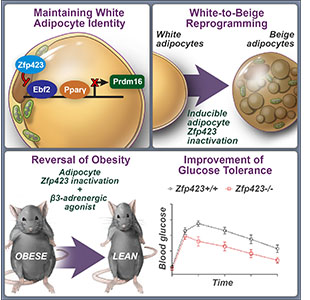
How white adipocytes suppress their thermogenic gene program has remained unclear. We have identified Zfp423 as a molecular brake on adipocyte thermogenesis through inhibition of Ebf2 activity. Inducible deletion of Zfp423 in obese mice triggers a white-to-beige adipocyte reprogramming and a reversal of obesity and insulin resistance.
Cell Metabolism. 2016 Jun 14;23(6):1167-84.
PubMed PMID: 27238639.
Graphics designed by Visually Medical.
Key Publications
Transcriptional control of preadipocyte determination by Zfp423.
Nature. 2010 PMID: 20200519
Fetal development of subcutaneous white adipose tissue is dependent on Zfp423.
Mol Metab. 2016 PMID: 28123942
Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program.
Cell Metab 2016 PMID: 27238639
Directing visceral white adipocyte precursors to a thermogenic adipocyte fate improves insulin sensitivity in obese mice.
Elife. 2017 PMID: 28722653
ZFP423 Controls EBF2 Co-activator Recruitment and PPARγ Occupancy to Determine the Thermogenic Plasticity of Adipocytes.
Genes Dev. 2021 PMID:34620682
2. Molecular Mechanisms Controlling Adipose Tissue Expansion and Remodeling in Adulthood.
The manner by which adipose tissue expands, or “remodels”, is a critical determinant of metabolic health in obesity. Pathological adipose tissue expansion is characterized enlarged adipocytes, a failure to recruit new adipocytes (adipogenesis), hypoxia, fibrosis, and pro-inflammatory macrophage infiltration. Healthy adipose tissue expansion (observed in the “Metabolically-Healthy” obese) involves recruitment of new adipocytes from resident precursors and lower levels of inflammation. This limits the burden on individual fat cells and prevents the acquisition of insulin resistance.
We have identified functionally distinct adipose progenitor cell subpopulations that regulate multiple aspects of healthy and unhealthy adipose tissue remodeling. We have identified PDGFRβ+ adipocyte precursor cells (APCs) that are activated to differentiate into white adipocytes in the setting of obesity. Using single-cell RNA sequencing and multi-omics technologies we have defined subpopulations of progenitor cells in murine adipose tissues. Ongoing efforts in the lab are focused on identifying the signals that activate these progenitor cell populations in obesity and elucidating the array of functions that these cells have on energy balance and nutrient homeostasis. Understanding the biology of adipose precursors will shed tremendous insight into the developmental origin of fat tissue and physiological regulation of adipose tissue distribution. The ultimate goal of our research is to use our understanding of adipocyte development to generate novel therapeutic strategies for the treatment of obesity and metabolic disease.
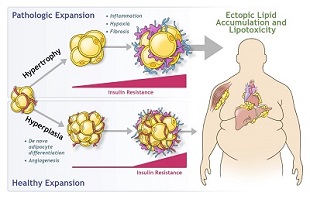
Expansion of white adipose tissue in response to caloric excess occurs through the enlargement of existing adipocytes (hypertrophy) and/or through an increase in adipocyte number (hyperplasia). Pathologic expansion through hypertrophy of adipocytes is associated with inflammation, hypoxia, and fibrosis, with early onset of insulin resistance. Adipocyte dysfunction leads to the deleterious spillover of lipids into non-adipose organs, termed “lipotoxicity”. Healthy expansion through hyperplasia of adipose tissue occurs through the recruitment of preadipocytes and de novo adipocyte differentiation. This occurs alongside with angiogenesis and prevents or delays the onset of both insulin resistance and ectopic lipid accumulation.
Molecular and Cellular Endocrinology. 2016 Oct 12.
PubMed PMID: 27743993.
Graphics designed by Visually Medical.
Key Publications
PDGFRβ+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice.
Cell Metab. 2016 PMID: 26626462
De novo adipocyte differentiation from Pdgfrβ+ preadipocytes protects against pathologic visceral adipose expansion in obesity.
Nat Commun. 2018 PMID:29497032
Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice.
Elife. 2018. PMID: 30265241
Perivascular mesenchymal cells control adipose-tissue macrophage accrual in obesity.
Nat Metab 2020 PMID: 33139957
Pathologic HIF1α signaling drives adipose progenitor dysfunction in obesity.
Cell Stem Cell. 2021 PMID: 33539723
Cold-responsive Adipocyte Progenitors Couple Adrenergic Signaling to Immune cell Activation to Promote Beige Adipocyte Accrual.
Genes Dev. 2021
Multilayered omics reveal sex- and depot-dependent adipose progenitor cell heterogeneity.
Cell Metab. 2022 PMID: 35447091
PUBLICATIONS
LINKS
Follow us @DukeGuptaLab
Learn More!
In this episode of Science Café, Dr. Gupta discusses the problem of obesity and the enigma of why the expansion of fat tissue can lead to diabetes and metabolic syndrome in some individuals but not others. He also highlights how “fixing our fat” can break the link between obesity and diabetes or even protect against obesity itself. Learn how fat tissue is not our foe, but rather our friend.
News Articles
- Texas researchers discover way to turn fat cells into energy-burning cells
- Researchers discover blood vessel cells that triggers harmful inflammation in obese mice
- Dr. Gupta Discusses Career Development and the Life of a Principal Investigator (2018)
MEET THE LAB



Former Members:
Research Technicians
Karen MacPherson (2012-2015)
Margaret Wang (2013-2014)
Napolean Busbuso (2016-2018)
George Elmquist (2019-2022)
Maggie Zumwalt (2020-2022)
Graduate/Medical Students
Chelsea Hepler (2014-2018)
Winner of the UTSW Nominata Award
Postgraduation: F32 funded Postdoctoral Fellowship, Bass lab, Northwestern University
Stephen Spurgin (2014-2015), UTSW Dean’s Research Scholar Fellowship Program
Postgraduation: Physician Scientist Training Program, UT Southwestern
Qianbin (Winnie) Zhang (2017-2022) UTSW Genetics, Development, and Disease Program
Postgraduation: Postdoctoral Fellowship, Broad Institute and Beth Israel Deaconess Medical Center, Claussnitzer and Rosen Laboratories.
Postdoctoral Trainees
Mengle Shao, Ph.D. (2014-2021)
Current Position: Principal Investigator, Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, CAS
Bo Shan, Ph.D. (2018-2022)
Current Position: Principal Investigator, Zhejiang University Institute of Translational Medicine
Jasmine Burrell, Ph.D. (2021-2022)
Current Position: Postdoctoral Fellow, Moorehouse School of Medicine, Badri Lab







